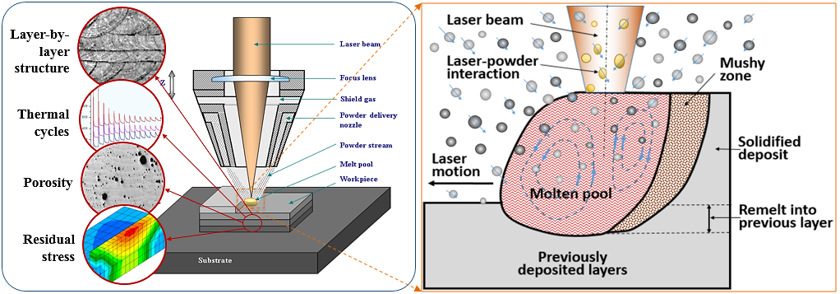
Before advanced materials or innovative fabrication methods can be used in high-technology applications, they must be developed and characterized – to push the boundaries, we must first learn where they are! To that end, the overall project of Materials Science begins with discovering new viable materials and learning how to use existing materials in new ways.
Magnesium Alloys
| The group is investigating the use of magnesium alloys in engineering applications where they have previously not been practicable. The advantage of magnesium alloys is that they are one of the lightest engineering alloys available, which means that they have great potential for automotive, aerospace, and portable electronics applications. The challenge of magnesium alloys, however, is that they are very brittle compared to materials like stainless steel or aluminum. This is because of the limited number of slip systems in magnesium’s hexagonal close packed crystal structure, as opposed to the cubic crystal structure of steel and aluminum. In an effort to extend the applicability of magnesium as an engineering material, characterization research of magnesium alloys is ongoing. The goal is to better understand the connection between the microstructure and bulk mechanical properties of these materials so as to learn how to enhance their desirable traits and, specifically, reduce their brittleness. An important part of this characterization effort is material deformation testing with in situ imaging. That is, while undergoing deformation tests these materials can be imaged with transmission & scanning electron microscopes (TEM & SEM), providing a real-time look at the internal deformation of the materials and, thus, important insight into the microstructure—mechanical property relationship. |
High Entropy Oxides
| Following the explosion in the last decade of the field of high entropy alloys, alloys composed of equimolar amounts of five or more metals, attention is turning to the potential of similar developments in ceramics. The JMS group conducts research on the possibilities presented by these high entropy oxides (HEO). High entropy stabilization allows for the creation of material systems with complexities greater than was once thought possible. This allows for the exploration of materials which include greater numbers of elements, thereby expanding the available “composition space” in which to develop materials with interesting properties. Due to high entropy stabilization, HEOs exhibit the behavior of having a reversible phase transformation, moving very easily between two phase states with minimal processing. For example, an HEO system can radically change its phase state with just a heat treatment and, crucially, reverse such a change in a similar manner. The possible industrial applications are considerable, but right now HEOs are in their infancy and research on them consists primarily in looking at the fundamentals of these materials. In engineering their microstructures to look at the fundamental science underlaying their properties, the question to be answered is: “how does the microstructure affect the phase transformation?” The JMS group is attempting to answer that question. An HEO studied by the group is one composed of oxides of cobalt, copper, magnesium, nickel, and zinc: (Co, Cu, Mg, Ni, Zn)O. To produce these materials, ceramic nano-powders are mixed with a planetary ball mill and then consolidated into a part via sintering – primarily spark plasma sintering (SPS), as it is good at retaining nanoscale microstructures. The HEO is then submitted to heat treatment and quenching. Finally, X-ray diffraction (XRD) is used to examine the phase states of the material. An intriguing development is that HEO materials with very fine (nano) scale microstructures have markedly different phase behavior than the same material with coarser microstructures. |